UTI qPCR Testing
Molecular Testing Labs can identify multiple pathogens and antibiotic resistance markers that can be difficult to grow in culture to assist in treatment options for complex or recurrent urinary tract infections (UTIs). We can provide next-day results with high accuracy of pathogenic DNA and resistance markers to help providers make the best-informed decision for their patients.
Tailored Solutions for UTI Testing
Molecular Testing Labs UTI testing utilizes state-of-the-art qPCR technology and automated extraction methods to potentially detect up to 25 pathogen targets associated with complex urinary tract infections (UTIs), including four sexually transmitted infections (STIs), and 22 pathogen-related antibiotic resistance targets (ABR).
Clinic Collected and Mailed

Our test method is highly sensitive, with a 98.9% detection* rate of known positive samples, and 100% specificity* during validation. Let Molecular Testing Labs’ UTI testing provide you and your patients with fast and reliable results.
Our reports are easy-to-read, provide antibiotic treatment recommendations, and can conveniently be received either via a protected online portal or through an electronic medical record (EMR) system. Results are generally available on the same day the sample was received for testing, helping to ensure your patients get treated quickly and efficiently.
MTL has top-of-the-line technology providing you with fast and reliable results for patients with complex or persistent UTIs.
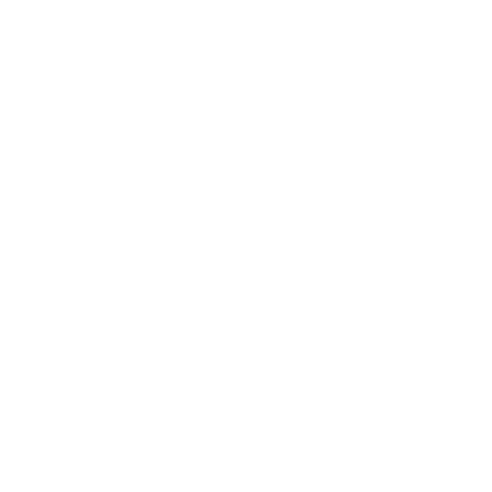
98.9%*
Test accuracy
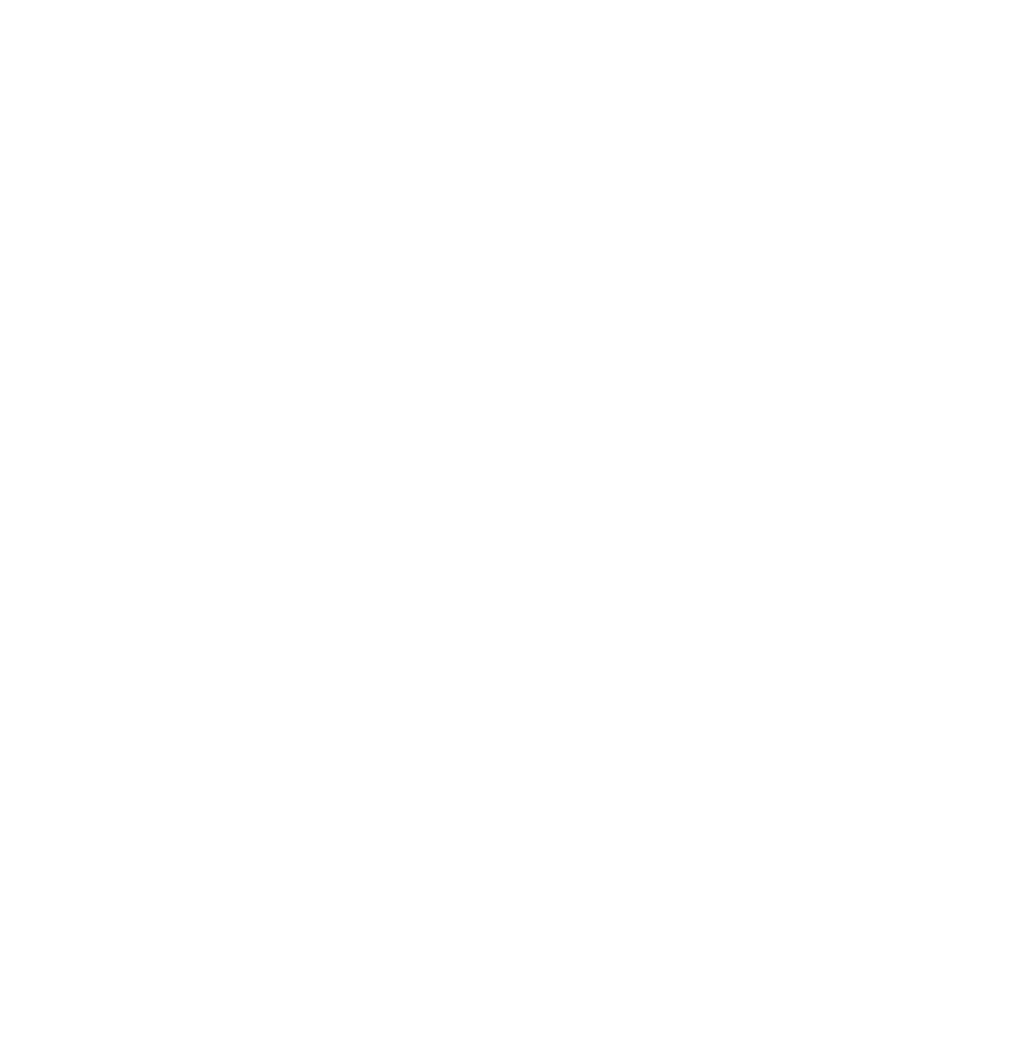
24 hrs
Turnaround times as
fast as one day
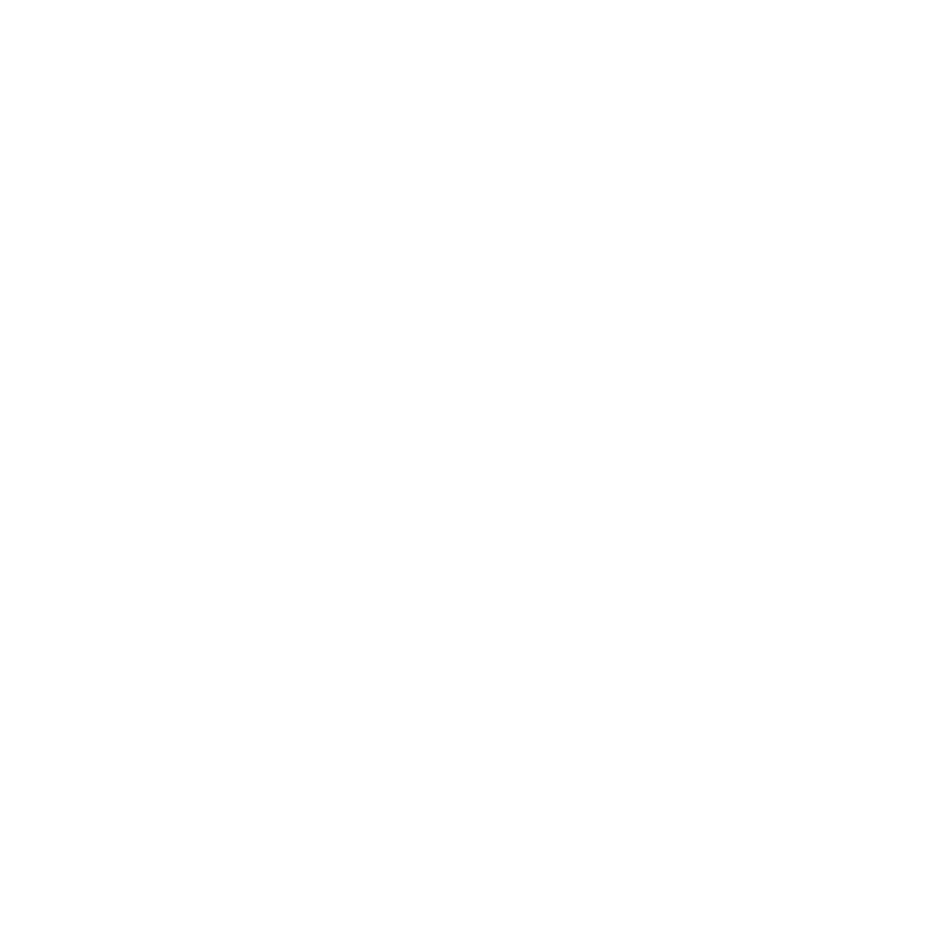
25
May detect up to 25 specific pathogens
* This test was developed, and its performance characteristics were determined, by Molecular Testing Labs (MTL). This test has not been cleared or approved by the U.S. Food and Drug Administration as it is validated as a Laboratory Developed Test. MTL is regulated and qualified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA) to perform high-complexity testing. Pursuant to the requirements of CLIA, MTL has established and verified this test’s accuracy and precision. Validation documents may be provided following disclosure requests and upon execution of a non-disclosure agreement.
MTL’s scientific team support, providing answers to your clinical questions.
Our Urology Business Development Team
Interested In Learning More?
Let us Empower you.









