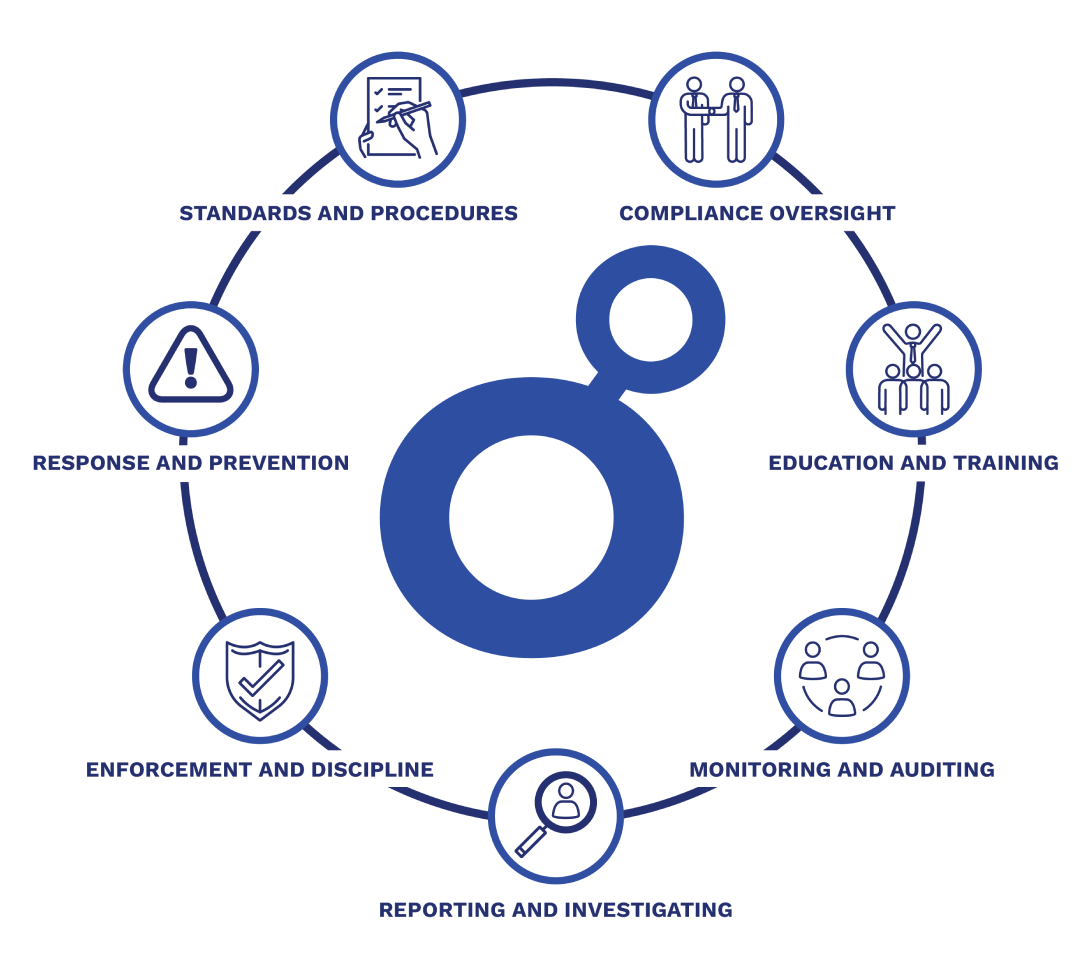
OUR DEDICATION TO
COMPLIANCE & ETHICS
COMPLIANCE & ETHICS
Compliance
We are committed to establishing ethical standards and conducting ourselves with the utmost integrity and discretion.
Unity
We are stronger when we work collaboratively and treat employees equitably regardless of their position in the company.
Innovation
We use science, technology, creativity, and persistence to solve problems.
Integrity
We speak and act from a position of honesty to honor confidentiality and build trust.
Partnership
We are your scientific team providing accessible and innovative laboratory solutions.
Agility
We challenge the status quo with open minds, focus and speed.
Molecular Testing Labs validates Assays with the feasibility of collection in mind.
Molecular Testing Labs is a CAP accredited and CLIA licensed lab. We apply the maximum level of control to obtain the most consistent and highest quality results.
Report a Concern
As a part of Molecular Testing Labs’ Compliance & Ethics Program, we provide a reporting hotline. The purpose of this service is to ensure that anyone wishing to submit a report has the ability to do so. Reports may be submitted anonymously.
The following toll-free numbers and other methods of reporting are available 24 hours a day, 7 days a week.
• English: (855) 400-6002
• Spanish: (800) 216-1288
• Website: https://www.lighthouse-services.com/moleculartestinglabs
Interested in Learning More?
Let us Empower you.